Psychedelics & Drug Policy: Next Steps
- yourbrainscience
- Aug 31, 2022
- 6 min read
Updated: Sep 27, 2022
The DOI Case
On Friday August 26, 2022, the Drug Enforcement Administration withdrew proposed Schedule 1 status for 2,5-dimethoxy-4-iodoamphetamine (DOI) and 2,5-dimethoxy-4-chloroamphetamine (DOC).
The notice states that “The Drug Enforcement Administration (DEA) is withdrawing a proposed rule that was published in the Federal Register on April 11, 2022, which proposed to place two phenethylamine hallucinogens in Schedule I of the Controlled Substances Act.”

We posted a couple weeks ago about the recent DEA withdrawal of another proposed rule to schedule five tryptamine psychedelics. The unprecedented withdrawal of that rule happened just weeks before the original scheduled pre-hearing for DOI and DOC. The August pre-hearing was pushed back 30 days due to filing from Panacea Plant Sciences challenging the DEA not following proper procedure for posting dates publicly. Then, on August 26th, just one month before the rescheduled pre-hearing, the proposed rule was withdrawn.
Official notice of withdrawal from the DEA (right)
The last blog we wrote about these cases focused a lot on how the DEA has the power to regulate psychedelics, but this post is going to focus more on the lack of evidence for scheduling and what these withdrawals mean for the future.
The challenging of the DEAs proposed rule came full force. The large community of researchers and students logged individual public comments, as well as as signed petitions circulated by VCU students and Michael Cunningham of Gilgamesh Pharmaceuticals. Other companies, such as Panacea Plant Sciences, filed a request for hearing. Co-chairs of the Students for Sensible Drug Policy (SSDP) Scientific Policy Council, Lindsey Galbo (Wake Forest University School of Medicine) and Elijah Ullman (Emory University) and Danny J. Lustberg, PhD, along with Anousheh Bhakti-Suroosh (Salk Institute), and Alaina Jaster (Virginia Commonwealth University) filed a motion with the DEA for a hearing on the manner to prevent the proposed Schedule 1 status from going forward.
Why do we care?
DOI is an unscheduled high affinity, high potency partial agonist for the serotonin ( 5-HT2A) receptor, that is used mainly in scientific research. DOI has been very rarely used recreationally, unlike one of it's analogue DOM. DOI has been extensively researched for it's pharmacology and its ability to produce measurable behaviors as a proxy of psychedelic action in animals. DOC, also on the scheduling docket, is a close derivative of DOI and DOM, but is less common.
Despite the use of DOI as an important research tool, the DEA still attempted to claim that this compound has "high abuse potential" and poses a public health concern. The evidence provided by the DEA was found to be less than substantial to justify Schedule I status.
One of the main assays used by the governing bodies to analyze drugs for scheduling is the drug discrimination task. This task has been historically considered the "golden standard" for identifying abuse potential. The paradigm involves training an animal to press a lever in response to how they "feel" when injected with a known drug, like LSD. After the animal is trained, the researcher will give the animal a different drug to see if the animal can identify whether the drug in question is similar to the one they were trained to press the lever with. The animal is not administering the drug to itself, or facilitating any rewarding stimulation by pressing the lever. They are simply just associating their response to one drug with another.
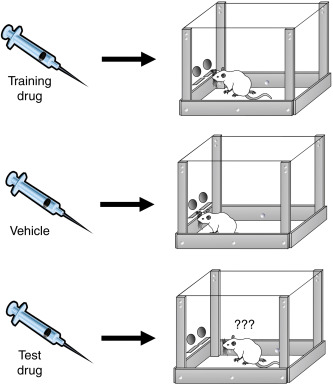
The task is more telling of how compounds are pharmacologically similar, but since the compounds typically assessed using this paradigm are already considered “drugs of abuse” by the governing bodies, there is no way for compounds to be tested in this paradigm and not be considered “drugs of abuse.”
Photo from B.T. Burrows et al (2017) Chapter 71- Synthetic Cannabinoids: a Summary of Selected Phenomena With Respect to Behavioral Pharmacology and Abuse Liability. Behavioral Pharmacology and Abuse Liability. Handbook of Cannabis and Related Pathologies.
What does this mean for the future?
Well, the optimist in all of us would like to think this victory indicates a shift in how the DEA will handle psychedelics. But it's all going to depend on what they do next. In the statement regarding the withdraw of the proposed rule for DOI, they state they will be
"publishing a new proposed rule using an amended procedure."
What this new procedure is, we can only speculate but there are some ideas about how the DEA will go about psychedelic drug scheduling as we move forward. One huge factor to this is the Biden administration stating they are hoping to approve MDMA and Psilocybin for psychotherapy within the next two years which will be headed by a task force of government officials. This huge announcement happened around the same time the tryptamine case was thrown out. With the changing landscape on psychedelics and their medical uses we can expect some changes in policy. What could that look like?
Continued bifurcated scheduling of compounds like we see with medically approved compounds based off "drugs of abuse." This is when the drugs, substances or specific chemicals used to make a preparation are classified differently depending on the "acceptablility," whether that be medically or socially. For example: heroin is schedule I, oxycodone is schedule II, Tylenol with codeine is schedule III and tramadol is schedule IV, but they are all classified as opioids. That is a perfect example of this scheduling concept.
Placing psychedelics and new schedules in schedule IV. This would be optimal because this lends to the idea that these compounds have medicinal value and have low potential for abuse. While DOI and DOC are not approved for medical use, but neither is etizolam, a compound related to benzodiazepines. Psilocybin has also been predicted to be put into Schedule IV, so this would be consistent.
The DEA comes back with the amended procedure, that could quite possibly have what they consider more evidence to schedule compounds at schedule I as originally proposed. This is probably unlikely because the current positive publicity for psychedelics and backing by congress and government officials would make severe scheduling and therefore the DEA very unpopular and not highly favored.
New Language Moving Forward
Something to think about in the coming days, weeks, months or even years moving forward is how we talk about drugs. The current process of scheduling drugs and the CSA relies heavily on the assumption that all drugs are drugs of abuse and all drugs of abuse are bad.
But aren't medicines drugs and aren't medicines good? Why is alcohol and nicotine socially acceptable but heroin and cocaine aren't? Why are psychedelics in the same schedule as heroin? Why hasn't THC (cannabis) been rescheduled knowing it has medical use?
There is a movement to talk about how people use drugs and how we talk about folks experiencing addiction. There are people who use drugs recreationally, spiritually and medicinally without any problems with addiction, but there are a subset of folks who fall victim to substance use disorders. While the use of drugs is always a choice, sometimes the brains response to drugs and a combination of genetic and environmental factors produce susceptibility to developing an addiction, which then becomes problematic to the persons quality of life. The use, misuse and abuse of substances is shifting to be thought of more as a spectrum rather than a black and white concept. If you asked 5 people today what the difference between use, misuse and abuse is, they would most likely all have a different answer. This lack of definition or understanding of drug use as a continuum can cause issues with policy because one policy maker may think that misuse only represents those who use prescriptions off-label, one may consider recreational drug use as misuse and addiction as abuse, while another may consider any non-socially acceptable drug use as abuse.
In order to form better and cohesive policy that is evidence-based, the scientific community needs to work with the public to inform policy makers on proper language.
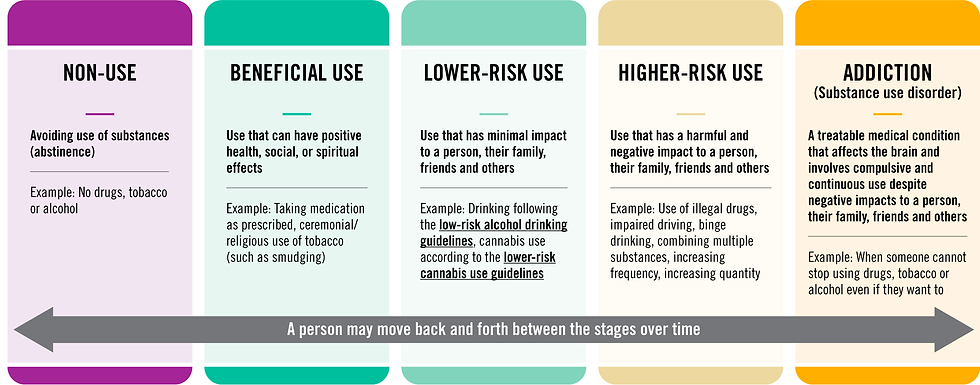
Infographic containing an example of the spectrum of drug use from the Government of Canada. Note: this is not the optimal spectrum, just an example of where the language is moving toward.
In Conclusion
We have been successful for now in deterring the DEA from further scheduling multiple psychedelics this year. That is something to celebrate! But we must keep in mind, there is a long way to go to fully reform how drug policy and regulation is handled in America. If you are interested in advocating for better policy check out your local area for organizations or consider forming a chapter of Students for Sensible Drug Policy, Students for Safe Drug Use, Decriminalize Nature or Drug Policy Alliance groups.

Comments